Genetically engineered animals in the food supply

Can you tell which ones are genetically engineered?



Genetically engineered animals and their products, most notably food products, are closer to reaching the market now that the Food and Drug Administration has released draft guidance on their regulation.
The FDA intends for the guidance document, which the agency released Sept. 18 for comment, to clarify its regulatory authority and the requirements and recommendations for developers of GE animals and products from GE animals.
"This is a cutting-edge technology that has significant implications, including real benefits, not just for human health, but also for animal health, such as developing disease-resistant animals," said Dr. Bernadette M. Dunham, director of the FDA Center for Veterinary Medicine.
The AVMA responded to the FDA guidance with a statement that noted potential benefits of GE animals while urging stakeholders to keep animal welfare in mind.
"The development and appropriate regulation of this technology has widespread applications in advancing our knowledge of diseases, food safety, environmental conservation, and efficient food and fiber production," wrote Dr. W. Ron DeHaven, AVMA chief executive officer, in the AVMA response.
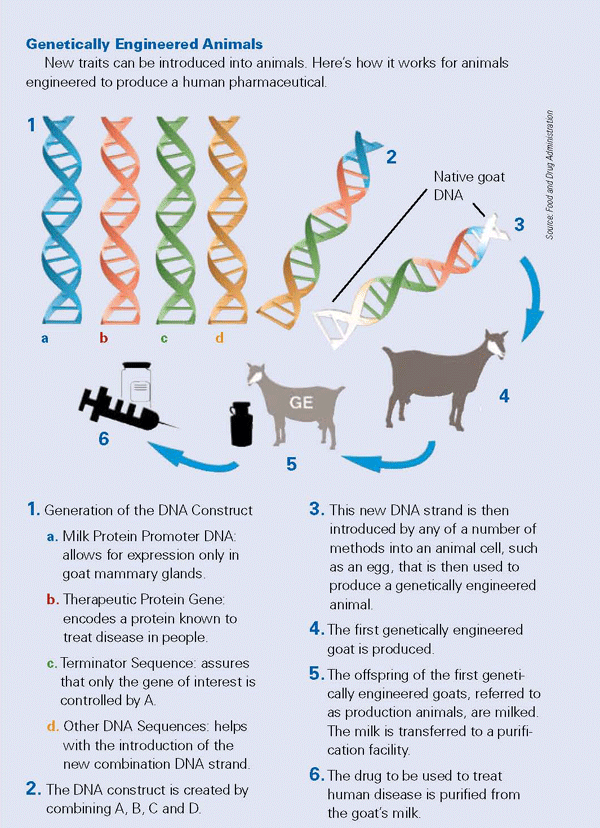
The agency will regulate GE food animals, such as pigs that produce omega-3 fatty acids. The CVM is working with other FDA centers on oversight of GE animals that produce biopharmaceuticals, such as goats whose milk contains proteins important in human medicine.
Even as the FDA moves forward, some scientists and the general public have concerns about GE animals. About 56 percent of Americans oppose research into genetic modification of animals, according to a 2005 survey through the Pew Initiative on Food and Biotechnology.
FDA guidance
Nevertheless, researchers have been creating GE animals since the early 1980s. The CVM has worked with developers of GE animals to ensure that food from these animals does not enter the food supply until or unless the FDA authorizes such use.
In the FDA guidance on regulation of GE animals, which focuses primarily on food animals, the agency defines GE animals as those containing a recombinant DNA construct to give them new traits. The FDA plans to regulate heritable rDNA constructs as new animal drugs under the Federal Food, Drug, and Cosmetic Act.
Developers of GE food animals would need to demonstrate that the rDNA construct is safe and effective in the GE animal, that food from the GE animal is safe for human consumption, and that the GE animal does not pose environmental risks.
"We must ensure that the products of biotechnology are safe before they are introduced into the marketplace and that they in fact do what they are intended to do," said Dr. William Flynn, CVM senior adviser for science policy, during a Sept. 18 stakeholders' teleconference on the FDA guidance.
The FDA intends to issue additional guidance on regulation of GE animals that produce biopharmaceuticals. Many other GE animals serve as models for human disease, but the FDA does not plan to regulate nonfood laboratory animals such as GE mice. The agency may also decide not to regulate some other nonfood GE animals. The FDA chose not to regulate glow-in-the-dark aquarium fish, for example, and the fish have since entered the market.
The bulk of the current FDA guidance document explains the process for developers of GE animals to file the paperwork for an investigational new animal drug or a new animal drug application.
"Developers of GE animals should contact CVM early in the development of their GE animal," according to the guidance. "Developers whose animals are already well under development also should contact CVM."
To complement the FDA guidance on GE animals, the Department of Agriculture is seeking input on actions and approaches to consider under the Animal Health Protection Act.
AVMA policy
Animal health is a central concern of the AVMA policy on "Creation and Use of Genetically Modified Animals," which the Executive Board approved in 2005 on recommendation from the Council on Research.
The policy supports creation of genetically modified animals as long as they do not harm the environment and the health and well-being of the animals remain preferential to human values and needs.
In part, the policy states: "Advancements made in sequencing the genomes of animals and improved technologies in functional genomics and biotechnology now present the opportunity to accelerate ongoing genetic improvements in animals at a pace and with a precision that is not possible by traditional selective breeding programs."
Dr. Kent Lloyd, associate dean for veterinary research at the University of California-Davis, is a member of the AVMA Council on Research and has worked with GE mice for about a decade.
"Transgenic technology is a very powerful technique for manipulation of the genome and phenotype of animals," he said. "Because such technology can have a dramatic impact on the health and welfare of affected animals, it is essential for the AVMA to have a position on the issue."
Dr. Lloyd said the FDA guidance seems well-conceived, thoughtful, and based on sound scientific judgment. Regarding the safety of each rDNA construct, the document notes the possible effects on animal health and recommends submitting a wide variety of relevant data.
The FDA guidance probably will not affect Dr. Lloyd's research and the research of other scientists who work with GE laboratory animals. Dr. Lloyd is a collaborator on the Knockout Mouse Project, which is helping create a collection of research mice missing individual genes.
Other researchers' reactions
Randall Prather, PhD, co-director of the National Swine Resource and Research Center at the University of Missouri-Columbia, works with GE pigs as models of human disease and also as a potential source of better human nutrition.
"We need to be able to feed an ever-growing world population and to try to alleviate human suffering," he said. "Genetic modification of animals is one way of reaching those goals."
Dr. Prather was a member of a team that recently created pigs that produce omega-3 fatty acids, which can be beneficial to human health and are found mostly in certain fish. Currently, he is helping develop GE pigs to serve as models of cystic fibrosis in humans. The FDA guidance, which Dr. Prather deemed to be reasonable, likely would apply to both projects because pigs are food animals.
Dr. Ina Dobrinski, director of the Center for Animal Transgenesis and Germ Cell Research at the University of Pennsylvania, also deemed the FDA guidance on GE food animals to be reasonable.
"They're going to be regulated on a case-by-case basis, and that seems sensible, given that we don't know how many animals will ever be made and stand any chance of entering the food chain," she said.
Dr. Dobrinski's current research focuses on improving strategies for creating GE pigs and goats that will serve as disease models or produce biopharmaceuticals. One goal is to target the incorporation of the rDNA construct more precisely.
While her animals probably will never enter the food supply, Dr. Dobrinski found it interesting that the FDA does not plan to require labeling of food from GE animals. Yet, she agreed with the agency's reasoning that labeling is not necessary if the food is essentially the same.
Public input
Others have expressed reservations about food from GE animals relevant to issues such as labeling, environmental risks, and morality. Members of the Consumers Union and Union of Concerned Scientists were among the participants in the stakeholders' teleconference regarding the FDA guidance on GE animals.
Michael Hansen, PhD, senior staff scientist with the Consumers Union, questioned the decision not to label food from GE animals. He argued that the food is inherently different because of the insertion of an rDNA construct, but FDA officials believe labeling is unnecessary for food from GE animals that is nutritionally equivalent to other food.
Doug Gurian-Sherman, PhD, senior scientist with the Union of Concerned Scientists, discussed possible threats to endangered species if any GE animals become feral. During the teleconference, FDA officials said the review process for each GE animal will include open meetings of advisory committees to allow for public input on environmental and other issues.
The 2005 Pew survey on public opinion of GE animals found that 64 percent of respondents favored a regulatory approach that balances the interests of consumers and food producers. About 61 percent said food from GE animals should enter the market only after the government determines it to be safe, although 21 percent of respondents said the food should not enter the marketplace under any circumstances.
The survey also asked consumers which reasons they supported for genetically engineering animals. About 40 percent of respondents supported producing chickens resistant to avian influenza and cattle resistant to bovine spongiform encephalopathy. Only 4 percent considered novelty pets to be a good reason for genetic engineering.
About 63 percent of survey respondents believed the government should consider morality and ethics in regulatory decisions about GE animals. Pew arranged a follow-up workshop in 2006 by subject experts, who concluded that: "While scientists have focused on the physical and biological risks of creating novel organisms, public concern about the genetic revolution has included from the start additional worries about disruptions in the natural order."
Workshop participants noted, however, that the FDA's mandate is to address scientific rather than ethical issues.